Introduction: Sudden and unpredictable onset of pain is one of the biggest contributors to the morbidity of sickle cell disease (SCD), thought to occur due to obstruction of microvascular blood flow by rigid sickle-shaped red blood cells. Autonomic mediated vasoconstriction in the peripheral vasculature, with resulting decrease in tissue perfusion, is likely a triggering mechanism for the onset of vaso-occlusive pain, while the autonomic nervous system is also implicated in the pathogenesis of chronic neuropathic pain. Our analysis of 212 polysomnograms in SCD subjects showed that greater magnitude of nocturnal vasoconstriction (Mvasoc) is predictive of increased frequency of hospitalizations for vaso-occlusive pain crises (VOC) in the subsequent years (Chalacheva et al., Am J Hematol, 2020). In order to further develop this finding into a biomarker of more imminent pain, we monitored nocturnal photoplethysmography (PPG) at home with a wearable wristband device to measure vasoconstriction activity and heart rate variability, along with a daily electronic pain survey.
Methods: 10 subjects with SCD were consented and underwent autonomic monitoring with a wristband device (Biostrap Inc) that uses an optical sensor to measure continuous PPG during sleep, with the raw PPG waveforms available from a cloud-based server. A unique biomarker (Mvasoc) was used to quantify the magnitude of vasoconstriction from the raw PPG signal, considering the frequency, magnitude, and duration of autonomic mediated spontaneous vasoconstrictions each night. Heart rate variability was derived from the pulse-to-pulse interval (PPI) of the PPG signal. Spectral indices of the PPI represent parasympathetic activity (high frequency power; HFP) and sympatho-vagal balance (Low to high Ratio; LHR = low frequency power(LFP)/HFP). Subjects concurrently documented daily pain incidence using an electronic text-based REDCap survey, with pain intensity measured on a scale of 1-10 if experiencing pain. Daily stress levels were also recorded on a scale of 1-10. Multivariate regression analysis was performed.
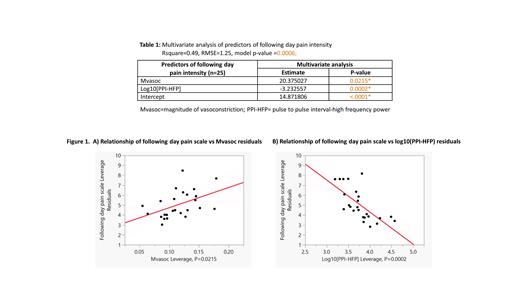
Results: The subjects had a mean age of 25 years (range 14- 40 years) with an average hemoglobin of 8.8 g/dl . A total of 68 usable nocturnal PPG recordings were made (3-16 nights per subject). Therewere 25 incidents of pain on the day following autonomic monitoring with Biostrap, with the pain intensity ranging from 3-8. Multivariate analysis of nocturnal autonomic and vasoconstriction parameters revealed that a lower HFP ( p =0.0002) and a higher Mvasoc ( p=0.02) both preceded a higher intensity of pain the following day (Table 1). These results are consistent with a greater parasympathetic withdrawal and a higher magnitude of peripheral vasoconstriction predicting higher intensity of imminent pain. Age, gender, hemoglobin, stress levels and LHR were not predictive of pain the following day.
Conclusion: Augmented nocturnal vasoconstriction and parasympathetic withdrawal noted prior to high intensity of pain imply that dysautonomia, with attendant changes in peripheral perfusion, has a significant role in pathogenesis of pain in SCD. Furthermore, autonomic vasoreactivity parameters were not significantly altered on the night after pain onset suggesting that the dysautonomia precedes pain. These results support the possibility of employing indices of nocturnal autonomic vasoreactivity as a predictive biomarker for imminent pain in SCD and provides a potential window for therapeutic intervention. This pilot study demonstrates the successful use of a wearable device PPG to remotely monitor nocturnal autonomic vasoreactivity and the ability to objectively quantify peripheral vasoconstriction responses. These findings will need to be applied in a larger cohort of SCD subjects with longer term monitoring for further validation and development of autonomic vasoreactivity as a predictive tool for pain in SCD.
Disclosures
Coates:Agios Pharmaceuticals: Consultancy; Chiesi: Consultancy; Bristol Myers Squibb: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal